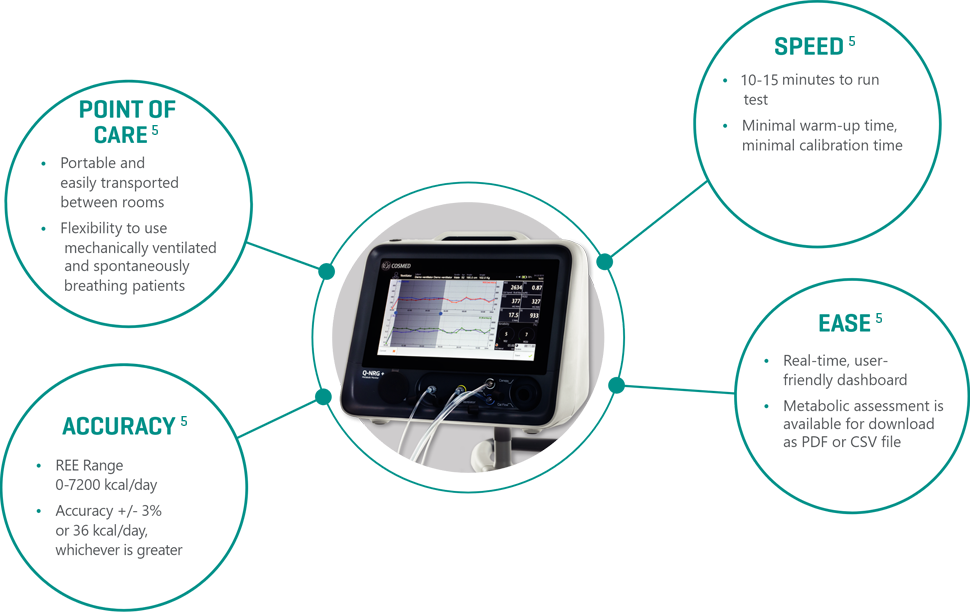
ESPEN and ASPEN guidelines recommend IC to accurately measure resting energy expenditure [REE] for critically ill, mechanically ventilated patients.1, 9
Predictive equations, while often used, are inaccurate up to 50% of the time in critically ill patients which may lead to over or underfeeding or increased risk of infection.2-3
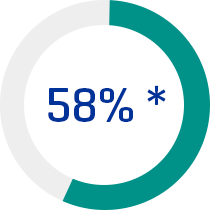
* Of prescribed nutritional needs

*Of recommended protein