Please click below to confirm you are a healthcare professional
Yes, I Confirmof US adults have chronic kidney disease3
Incidence increasing with age 44% of adults 70 and older3

of the US population use low-dose aspirin for CVD prevention4
of surgical patients are treated with anticoagulants and/or antiplatelets* 5
adults have liver disease6
adults will be diagnosed with cancer in their lifetime7

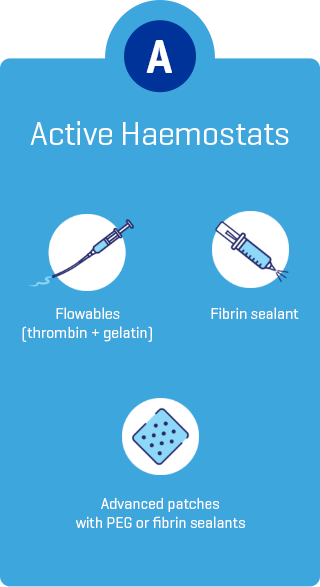
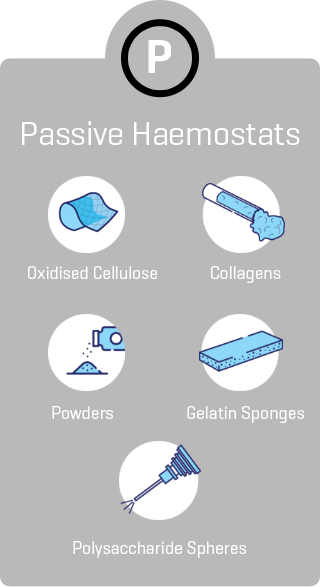
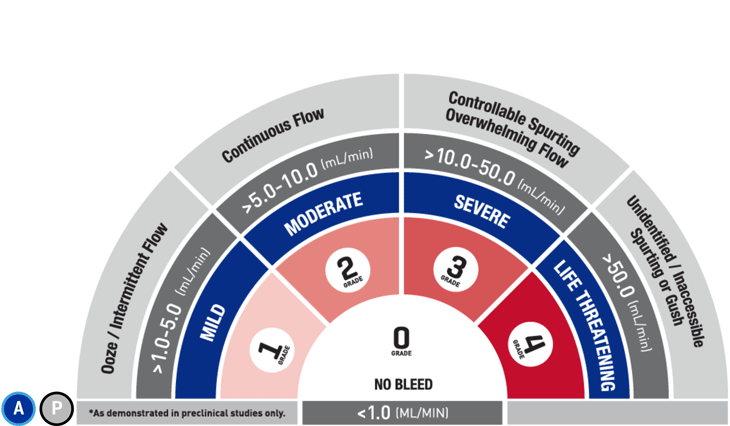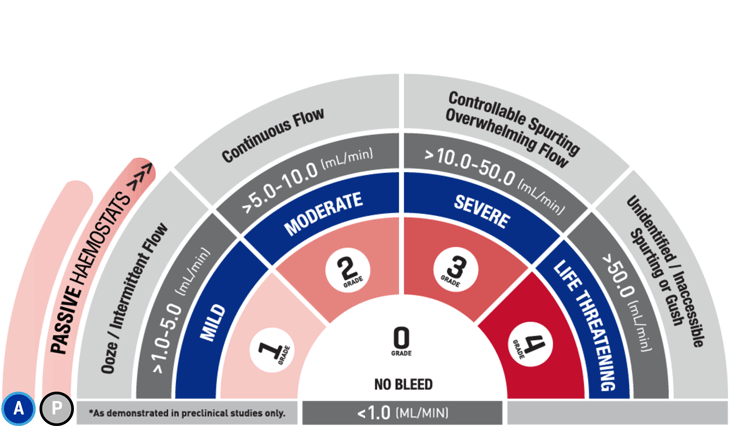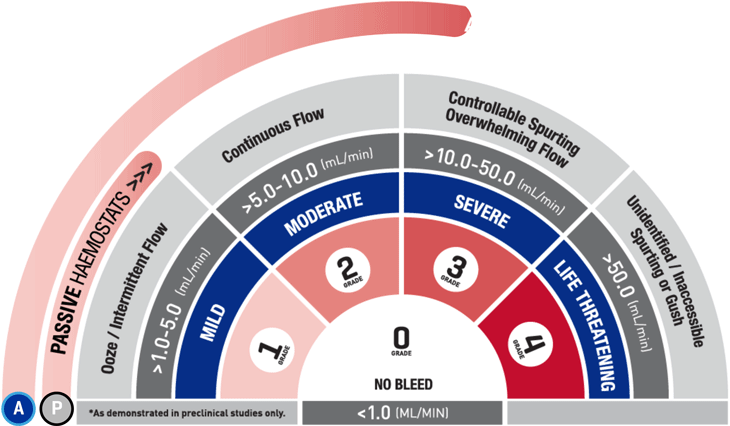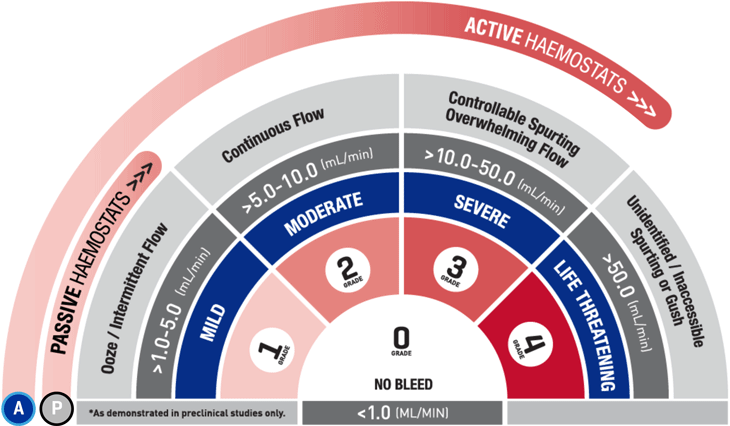
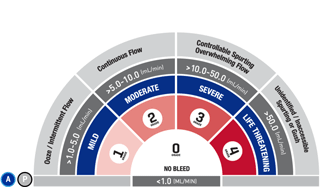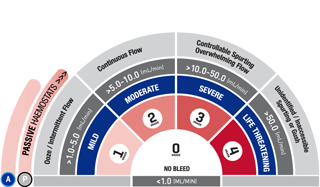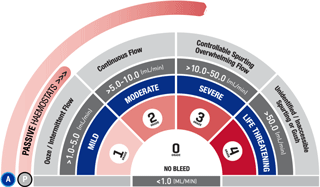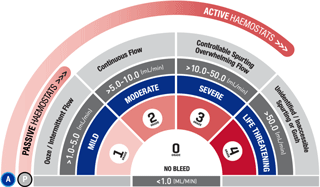
*As demonstrated in preclinical studies only.
1. Stokes ME, Ye X, Shah M, Mercaldi K, Reynolds MW, Rupnow MF, Hammond J. Impact of bleeding-related complications and/or blood product transfusions on hospital costs in inpatient surgical patients. BMC health services research. 2011 Dec;11(1):1-3. 2. Salive ME. Multimorbidity in older adults. Epidemiologic reviews. 2013 Jan 1;35(1):75-83. 3. Centers for Disease Control and Prevention. Chronic Kidney Disease Surveillance System. Available at: Chronic Kidney Disease (CKD) Surveillance System (cdc.gov) (Accessed August 2021) 4. Stuntz M, Bernstein B. Recent trends in the prevalence of low-dose aspirin use for primary and secondary prevention of cardiovascular disease in the United States, 2012–2015. Preventive medicine reports. 2017 Mar 1;5:183-6. 5. Baxter Data on File – Preliminary Results, Summary of Premier Data, 2018Q1 to 2019Q2 6. Centers for Disease Control and Prevention. Chronic Liver Disease and Cirrhosis. Available at: https://www.cdc.gov/nchs/fastats/liver-disease.htm (Accessed January 2020) 7. American Cancer Society. Facts & Figures 2019: US Cancer Death Rate has Dropped 27% in 25 Years, Jan 2019. Available at: https://www.cancer.org/latest-news/facts-and-figures-2019.html (Accessed January 2020 8. Corral M, Ferko N, Hollmann S, Broder MS, Chang E. Health and economic outcomes associated with uncontrolled surgical bleeding: a retrospective analysis of the Premier Perspectives Database. ClinicoEconomics and outcomes research: CEOR. 2015;7:409. 9. Slezak P, Keibl C, Labahn D, Schmidbauer A, Genyk Y, Gulle H. A comparative efficacy evaluation of recombinant topical thrombin (RECOTHROM®) with a gelatin sponge carrier versus topical oxidized regenerated cellulose (TABOTAMP®/SURGICEL®) in a porcine liver bleeding model. Journal of Investigative Surgery. 2020 Jan 17:1-7. 10. Nasso G, Piancone F, Bonifazi R, Romano V, Visicchio G, De Filippo CM, Impiombato B, Fiore F, Bartolomucci F, Alessandrini F, Speziale G. Prospective, randomized clinical trial of the FloSeal matrix sealant in cardiac surgery. The Annals of thoracic surgery. 2009 Nov 1;88(5):1520-6. 11. Bracey A, Shander A, Aronson S, Boucher BA, Calcaterra D, Chu MW, Culbertson R, Jabr K, Kehlet H, Lattouf O, Malaisrie SC. The use of topical hemostatic agents in cardiothoracic surgery. The Annals of thoracic surgery. 2017 Jul 1;104(1):353-60. 12. Lewis KM, Li Q, Jones DS, Corrales JD, Du H, Spiess PE, Menzo EL, DeAnda Jr A. Development and validation of an intraoperative bleeding severity scale for use in clinical studies of hemostatic agents. Surgery. 2017 Mar 1;161(3):771-81. 13. Baxter Data on File BWQ026-IS17/BWQ027-IS1 14. de Leval MR, Carthey J, Wright DJ, Farewell VT, Reason JT. Human factors and cardiac surgery: a multicenter study. The Journal of thoracic and cardiovascular surgery. 2000 Apr 1;119(4):661-72.
- For the UK reporting forms and information can be found at www.mhra.gov.uk/yellowcard.
- For Ireland report to the Health Products Regulatory Authority (HPRA) using a Yellow Card obtained from the HPRA, via the online system (www.hpra.ie) or by telephone on +353 (0)1-6764971.
- Adverse Events relating to Baxter products can also be reported direct to Baxter Pharmacovigilance on +44 (0)1635 206360, or by email to vigilanceuk@baxter.com.
- Drug or medical device product quality complaints relating to Baxter products can be reported directly to Baxter Healthcare Ltd:
- In the UK +44 (0)1604 704603, or by email to UK_SHS_QA_Complaints@baxter.com.
- In Ireland on +353 (0)1 2065500 or by email to shs_complaints_dublin@baxter.com.
- Alternatively please report directly to your Baxter Representative, who will take the details and forward to the Baxter Country Quality Assurance Team.